Dabo Krempus and colleagues have recently released a publication in the journal “Applied Thermal Engineering.” The study centers on utilizing mixtures as working fluids within ORC cycles, and the titled piece is “Utilizing Mixtures as Working Fluids in Air-Cooled ORC Bottoming Power Plants for Gas Turbines.” For their calculations, they employed the Asimptote Cycle-Tempo and FluidProp software. Teus van der Stelt from Asimptote also played a role as a co-author in the development of this publication. The article is openly available and can be viewed through the following link.
Abstract
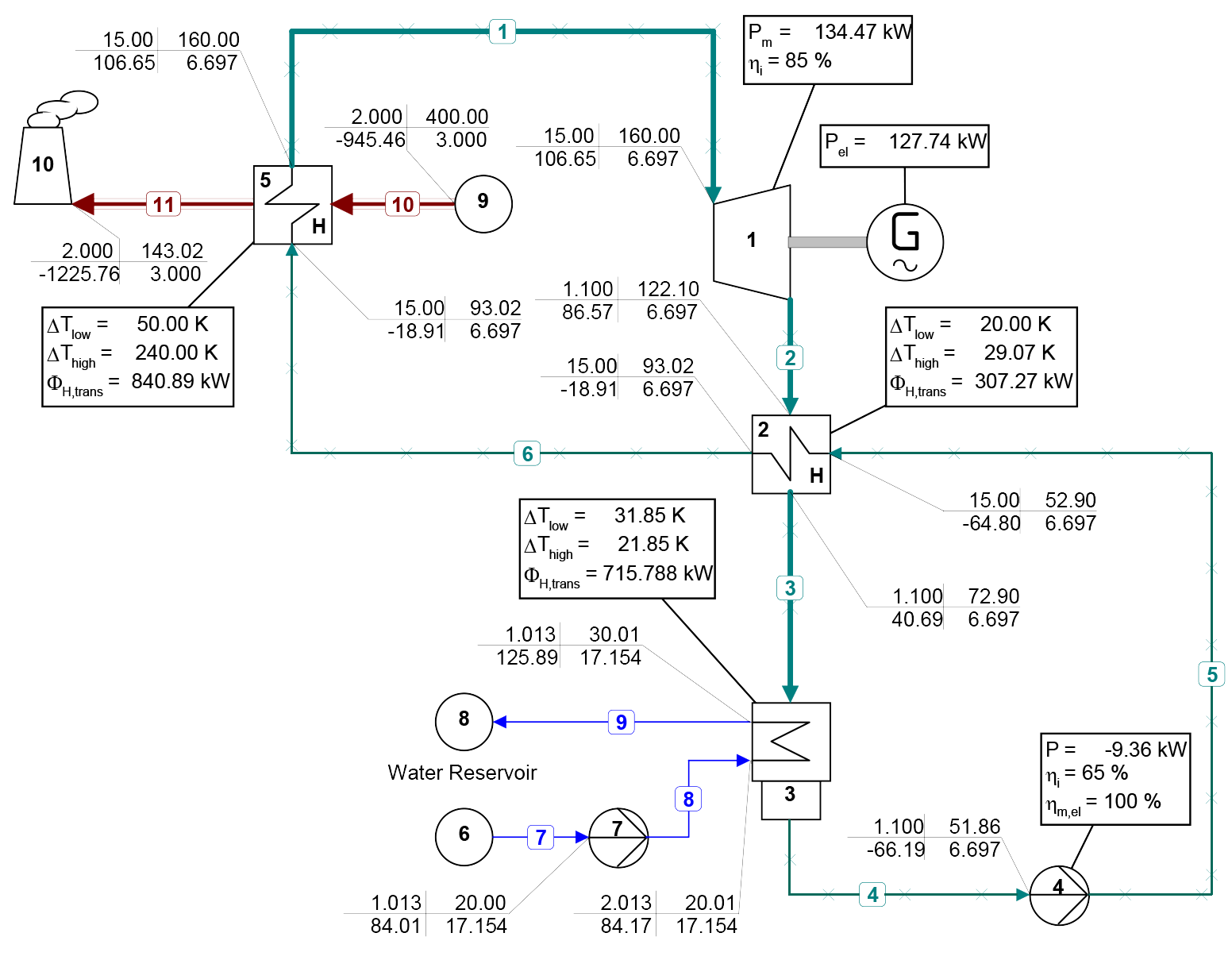
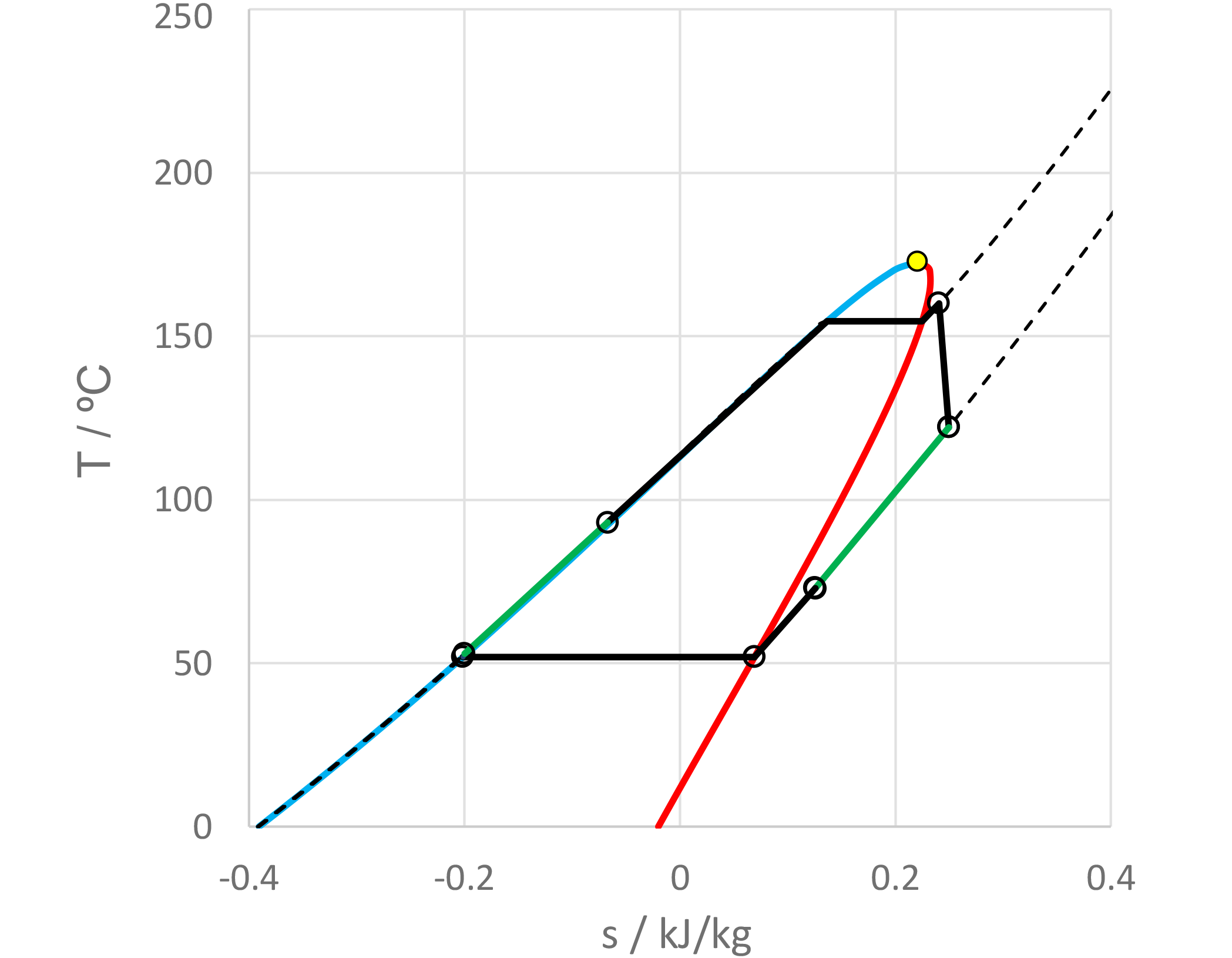
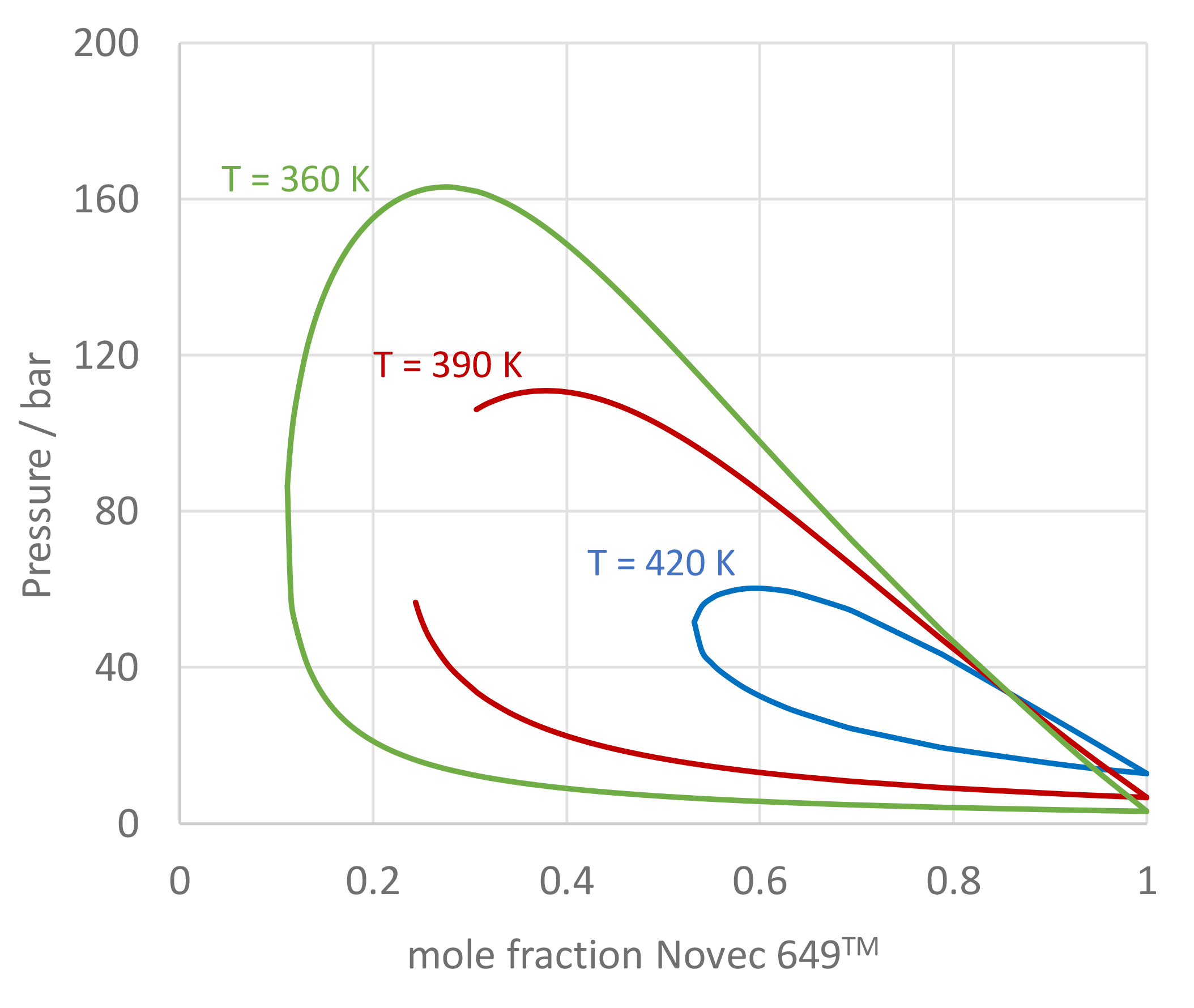
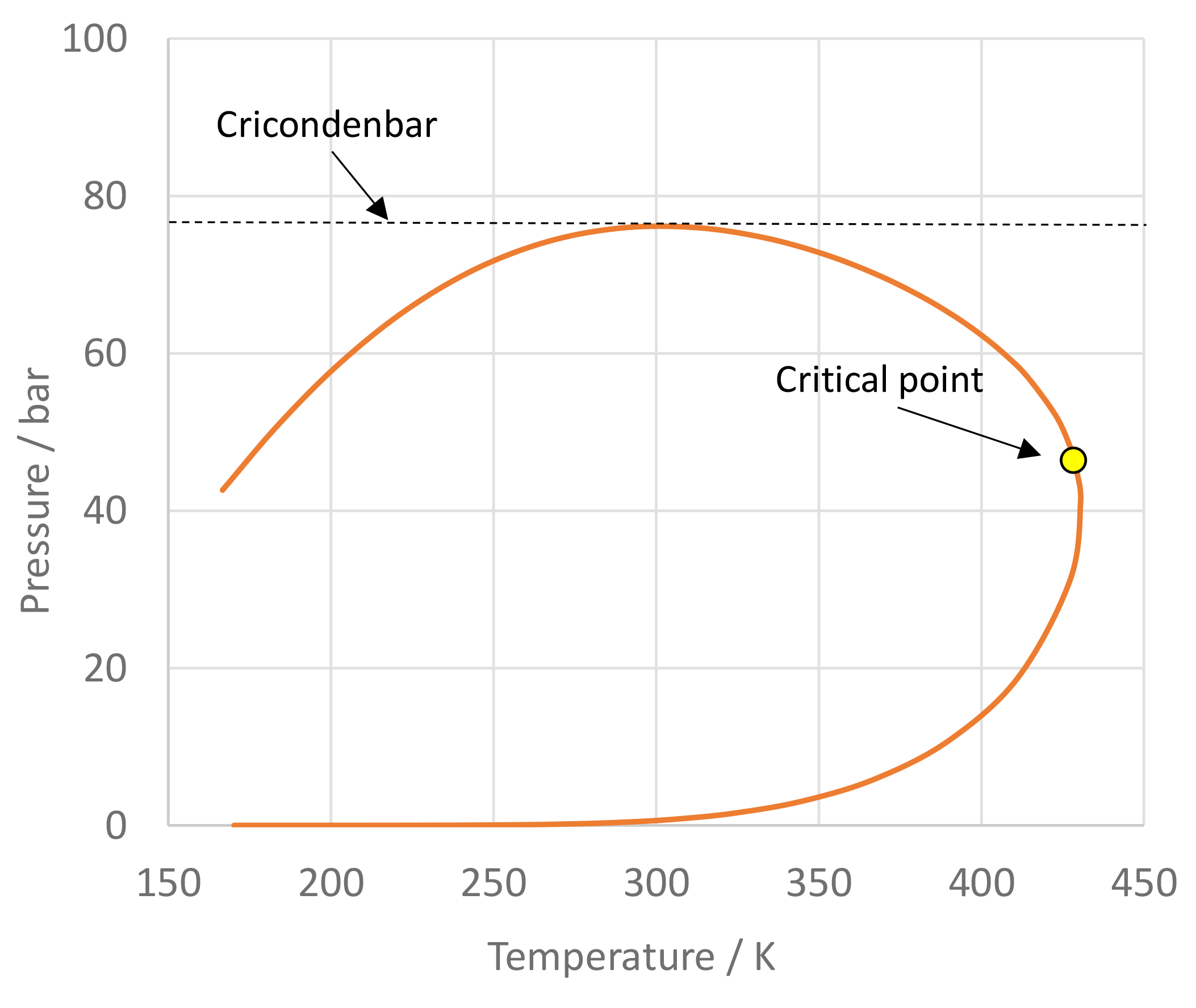
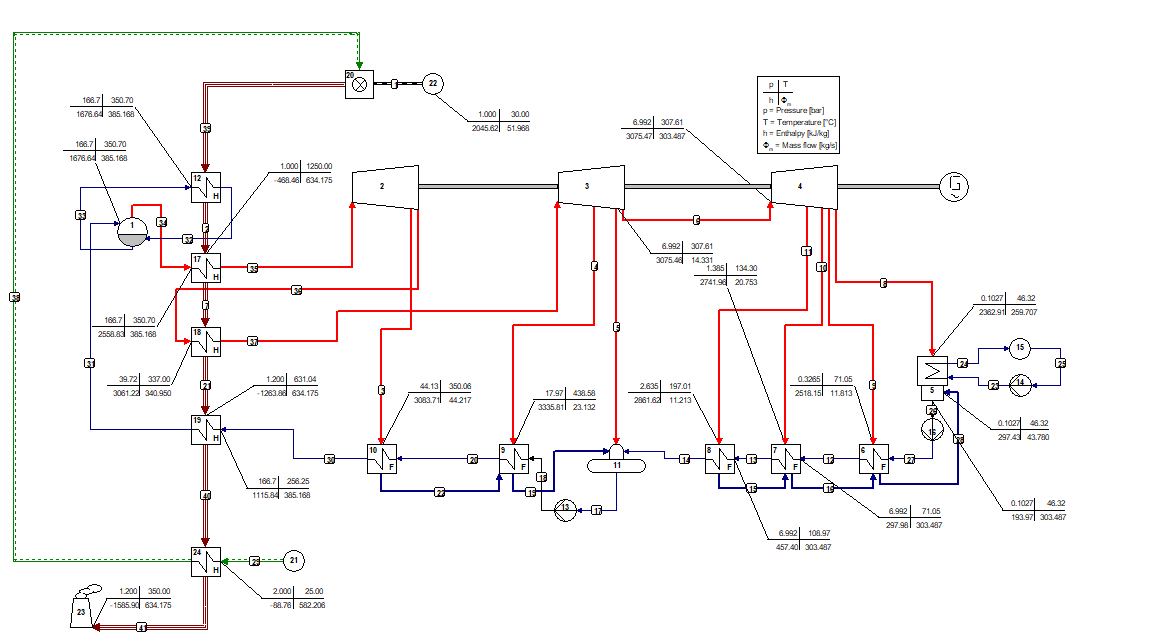
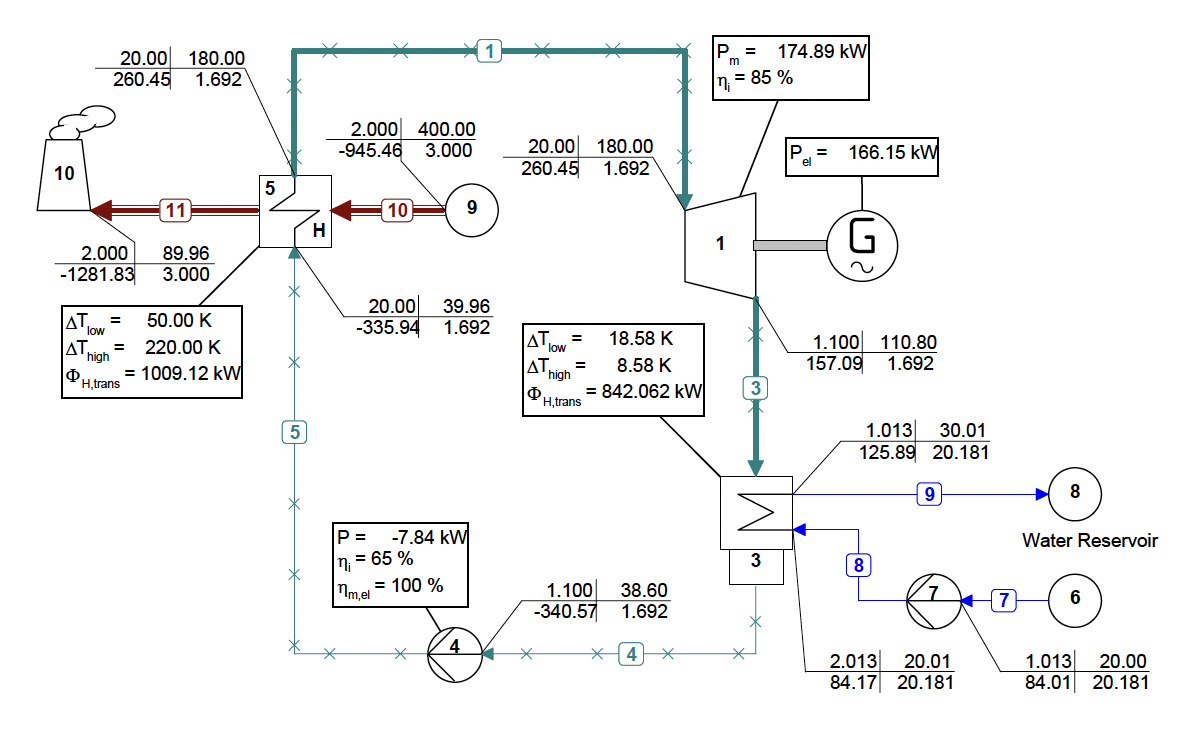
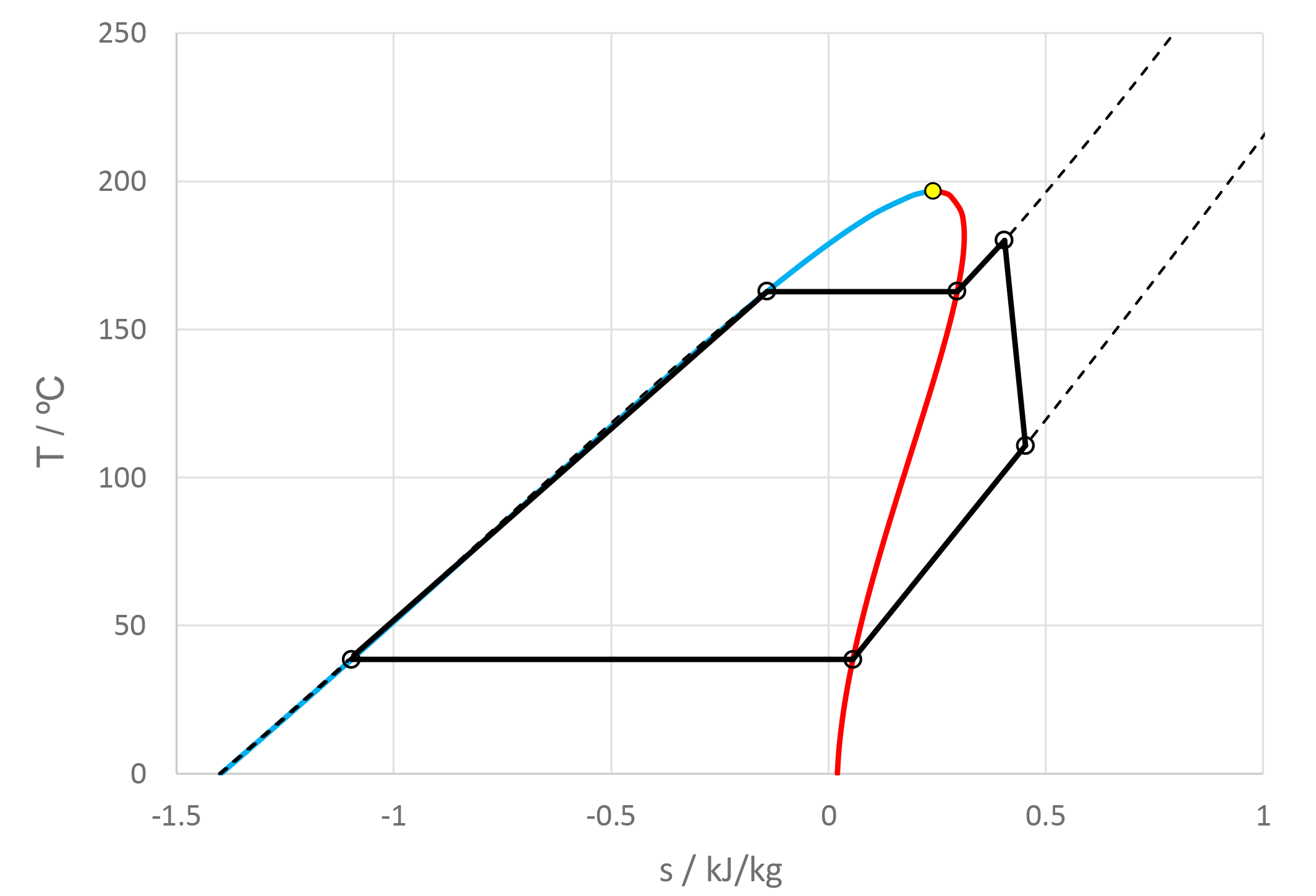
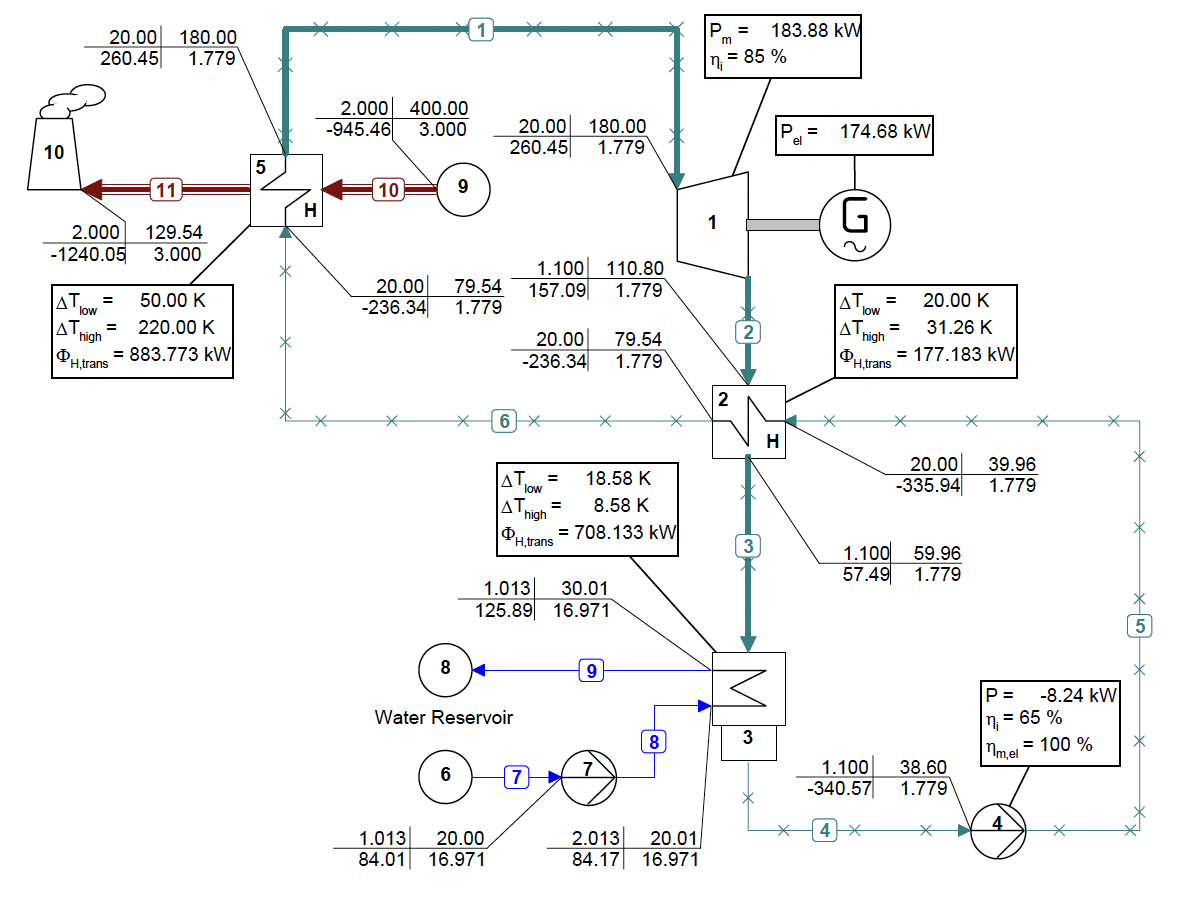
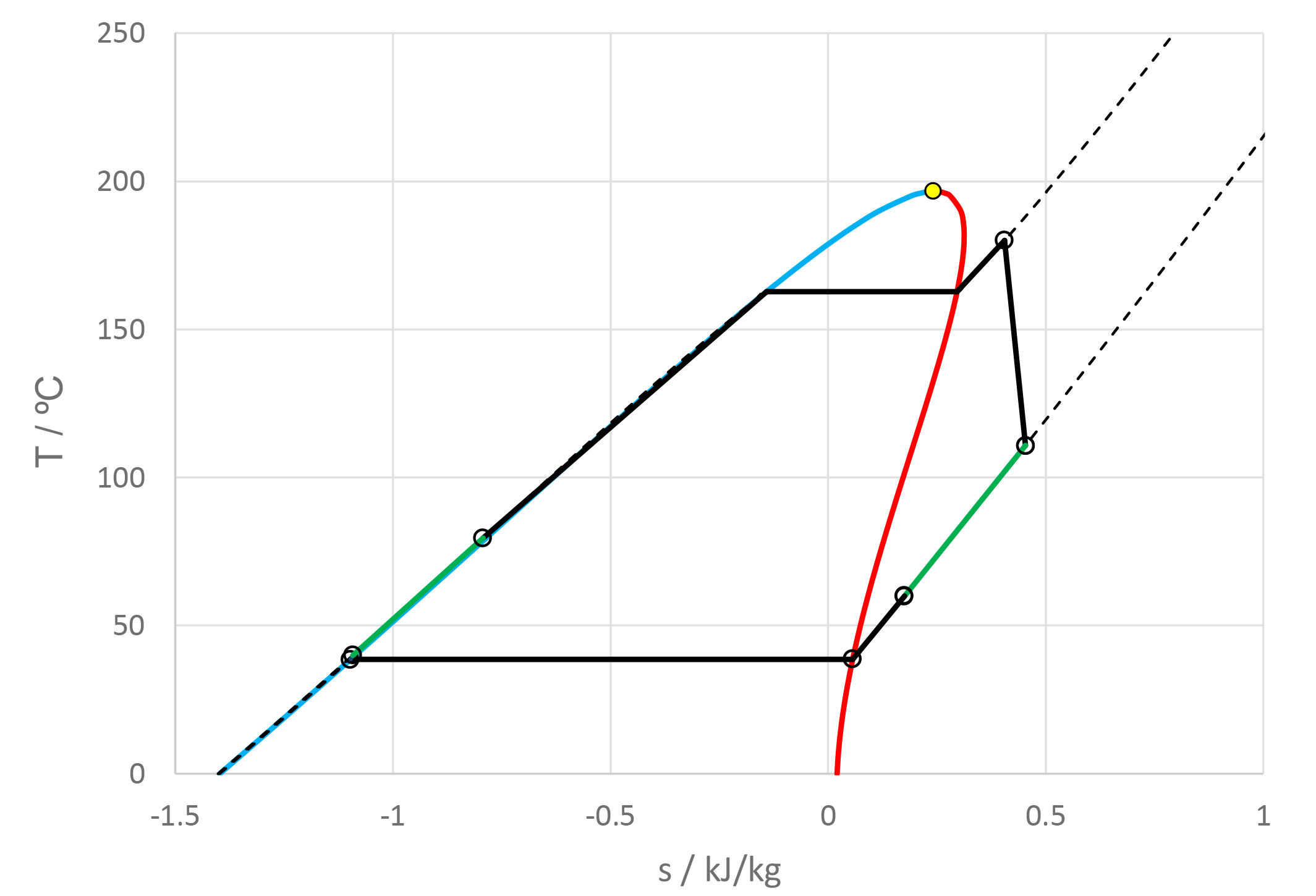
The use of mixtures as working fluids of organic Rankine cycle (ORC) waste heat recovery (WHR) power plants has been proposed in the past to improve the matching between the temperature profile of the hot and the cold streams of condensers and evaporators, thus to possibly increase the energy conversion efficiency of the system. The goal of this study is to assess the benefits in terms of efficiency, environmental (GWP) and operational safety (flammability) that can be obtained by selecting optimal binary mixtures as working fluids of air-cooled ORC bottoming power plants of medium-capacity industrial gas turbines. Furthermore, two thermodynamic cycle configurations are analyzed, namely the simple recuperated cycle and the so-called splitcycle configurations. The benchmark case is a combined cycle power plant formed by an industrial gas turbine and an air-cooled recuperated ORC power unit with cyclopentane as the working fluid. The results of this study indicate that binary mixtures provide the designer with a wider choice of optimal working fluids, however, in the case of the recuperated-cycle configuration, no improvement in terms of combined cycle efficiency over the benchmark case can be achieved. The split-cycle configuration leads to an increase of combined cycle efficiency of the order of 1.5%, both in case of pure and blended working fluids. Furthermore, for this cycle configuration the use of Novec 649 as working fluid is advantageous because it is environmentally and operationally safe, and it does not involve any penalty in terms of combined cycle efficiency if compared to the benchmark case. Additionally, the use of this fluid would lead to a more compact turbine, as the corresponding thermodynamic cycle would determine a turbine volume flow ratio that is half of the value of the benchmark case and a specific enthalpy difference over the expansion that is one fifth.